ZirChrom Newsletter vol. #3
Vol.# 3- “Improving the Selectivity and Peak Shape of Basic Analytes” (PDF)
Welcome to the third issue of ZirChrom’s electronic newsletter!
This newsletter issues approximately every 6 weeks and will address both general topics of interest to all chromatographers and specific features and benefits of ZirChrom’s family of ultra-stable zirconia-based HPLC phases. All issues discuss practical topics to help you improve your productivity and understanding of chromatography.
This issue is devoted to the unique benefits of using the chemical differences between zirconia and silica-based RPLC media to optimize selectivity and peak shapes of basic pharmaceuticals.
Type-A, Type-B and Type-Z Stationary Phases for HPLC----What’s the Difference?
The earliest silica phases used for HPLC, now called “Type-A” phases, contained hundreds parts per million of metal contaminants, which increased the Bronsted acidity of a significant fraction of the surface silanols. It is not possible to derivatize more than 40-50% of the 8 micromol/m2 of silanol sites even by extensive end-capping. As a consequence, when the residual silanol sites are in the ionized state (SiO-), they can interact with positively charged analytes such as basic pharmaceuticals. These strong sites, although few in number, cause a significant amount of retention and selectivity of basic analytes, but also very commonly cause peak tailing and wide peaks. Very importantly, due to the presence of the metal contaminants, these effects persist even at very low pH (<3).
Over the past decade, the purity of the silica substrate has been vastly improved (to only a few parts per million of metal impurities). Today’s silicas are so different that they constitute a completely new material (so-called “Type-B” phases). These improvements in purity and bonding greatly benefit the peak shape of many analytes, but also cause many end-capped, monofunctional (non polar embedded) ODS reversed phase columns based on Type-B silica to become rather similar in terms of selectivity. This data is completely consistent with John Dolan’s recent statement in LCGC (Ref. 1) concerning conventional ODS phases: “Generally, you can gain little by changing from one manufacturer’s column to another’s while keeping stationary phase type constant”.

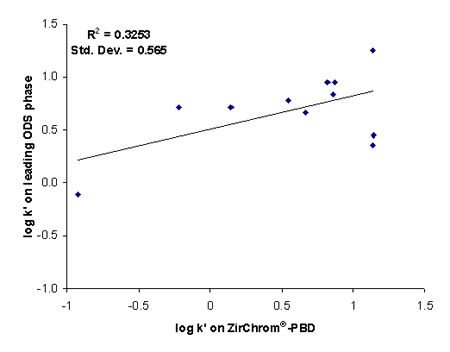
Zirconia-based RPLC media (such as ZirChrom PBD and PS) are inherently chemically very different from silica media in terms of their retention and selectivity for basic analytes. Although the retention on PBD and PS zirconia phases correlates very well with retention on ODS silica phases for non-ionizable polar and non-polar analytes, there are great differences in retention, and selectivity of basic molecules due to the chemical differences between silica and zirconia-based phases. In general, basic molecules are more retained on zirconia than on silica phases and in contrast more hydrophobic (less polar, nonionic) analytes are more retained on silica-based phases. As a consequence, the correlation between retention factors on Type-B silica phases and PBD or PS zirconia (“Type-Z”) phases is almost non-existent for basic analytes. Elution sequences are quite different.
It follows that two drugs that nearly coelute on typical Type-B silica phases will typically be separated on PBD or PS zirconia phases and vice versa. Thus PBD or PS zirconia and silica phases are highly complementary - the yin and the yang – so to speak for the separation of basic analytes. If two analytes are poorly separated on a Type B silica phase in accord with John Dolan’s statement it makes more sense to try a zirconia-based phase than another Type B silica phase, since most of these phases are similar (see Figure 2). Ref. 2 provides an excellent example of the tremendous changes in selectivity observed when one changes from a silica-based reversed phase to a zirconia-based reversed phase column.
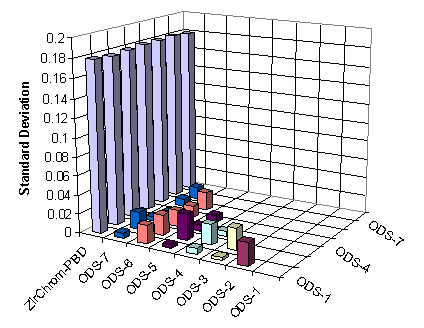
Because of this very strong difference in selectivity one of the situations in which we very strongly recommend that you give a ZirChrom PBD or PS column a try is in establishing a “stability indicating assay”. It is very important in such assays that all possible peaks even very small peaks (<0.1% of the active drug) be resolved. This inherently requires checking the separation with several columns of maximally different selectivity.
Buffer and pH Differences:
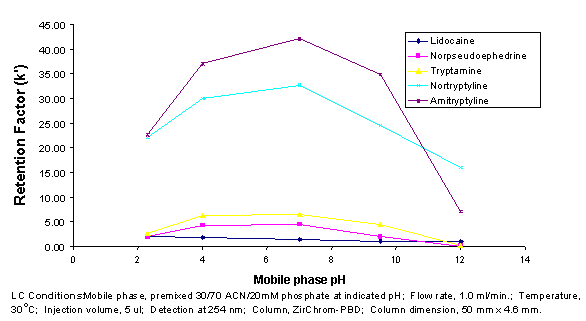
At pHs above the pKa of silica-based phases, the retention of amines increases as the pH is raised. The exact opposite phenomenon (see Figure 3) is observed with PBD and PS zirconia-based RPLC media, due to the fundamental differences in the retention mechanism. Consequently, the effect of a change in pH or a change in buffer type (e.g. acetate to phosphate) is almost always quite different when using zirconia and silica-based phases. Many of the interesting effects of buffer type and concentration hinted at here are described in detail in Ref. 3 below. This offers significant opportunities for adjusting selectivity on PBD or PS zirconia when the same variable fails to produce a good separation on a silica-based phase. An example of how buffer type can affect the selectivity of a separation of pharmaceutical molecules on ZirChrom’s PBD-zirconia phase is shown in Figure 4.
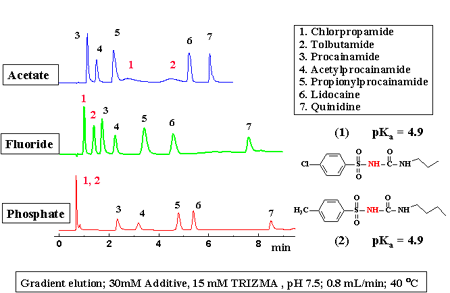
We typically recommend that chromatographers start method development in the neutral pH region (6 < pH < 8) with a 10-20 mM ammonium phosphate buffer at 40°C at a flow rate of 1-2 ml/min. However, due to their extraordinary inherent stability of ZirChrom’s PBD or PS RPLC columns can be operated indefinitely at low (< 2) and high pH (> 10) and at temperatures much above ambient (up to 150°C). We have yet to see any amine which does not give excellent peak shape at pH 12 in an ammonium phosphate or tetramethyl ammonium hydroxide buffer on PBD or PS zirconia phases. Our RPLC media can be used at very low pH with trifluoroacetic acid under LC-MS compatible conditions, but retention of very basic analytes tends to be low. Clearly, ZirChrom’s columns offer the chromatographer a very wide scope for optimization of selectivity and resolution.
TIP: We strongly suggest you check out our free Buffer Wizard tool for doing all the tedious calculations involved in preparing a buffer.
Loadability:
It is well known that the loadability of amines on typical ODS phases is considerably lower than that of nonpolar, non-ionized solutes. Again, the retention mechanism is so different on PBD or PS zirconia-based phases that the opposite is true. In fact ZirChrom’s PBD or PS phases typically show greater loadability of basic solutes than do typical ODS phases. This can greatly benefit the analysis of trace impurities in basic drugs and the isolation of such impurities for identification purposes.
Want More Information?
The above is a very brief overview hitting on only a few of the important benefits resulting from the chemical differences between silica and zirconia-based RPLC media. For more information on how to rapidly develop a separation by utilizing these differences please request a free copy of our Method Development Guide for New Users by email to support@zirchrom.com or FAX to 763-421-2319. We have also developed a slideshow on method development using ZirChrom’s RPLC phases. This slideshow can be downloaded here.
Literature Cited:
1. J. Dolan, “Resolving minor peaks.”, LC-GC, 20 ( #7) , p 598 (2002).
2. Y. Mao and P.W. Carr, "Separation of selected basic pharmaceuticals by reversed-phase and ion-exchange chromatography using thermally tuned tandem columns." Analytical Chemistry 73, 4478-4485, (2001).
3. Y. Hu, X. Yang and P. W. Carr, J Chromatography A, 968, 17-29, (2002)