ZirChrom Newsletter vol. #13
Vol.# 13- “Multi-modal Separations and Unique Selectivity using ZirChrom« HPLC Phases” (PDF)
Welcome to the thirteenth issue of ZirChrom's electronic newsletter. In this volume we feature the multi-modal capability of ZirChrom columns. Although the benefits of zirconia's unparalleled stability are clearly apparent, the more subtle value of the multi-modal nature of zirconia may in fact be the most important factor when considering a zirconia column for your application.
WHY ZIRCONIA?
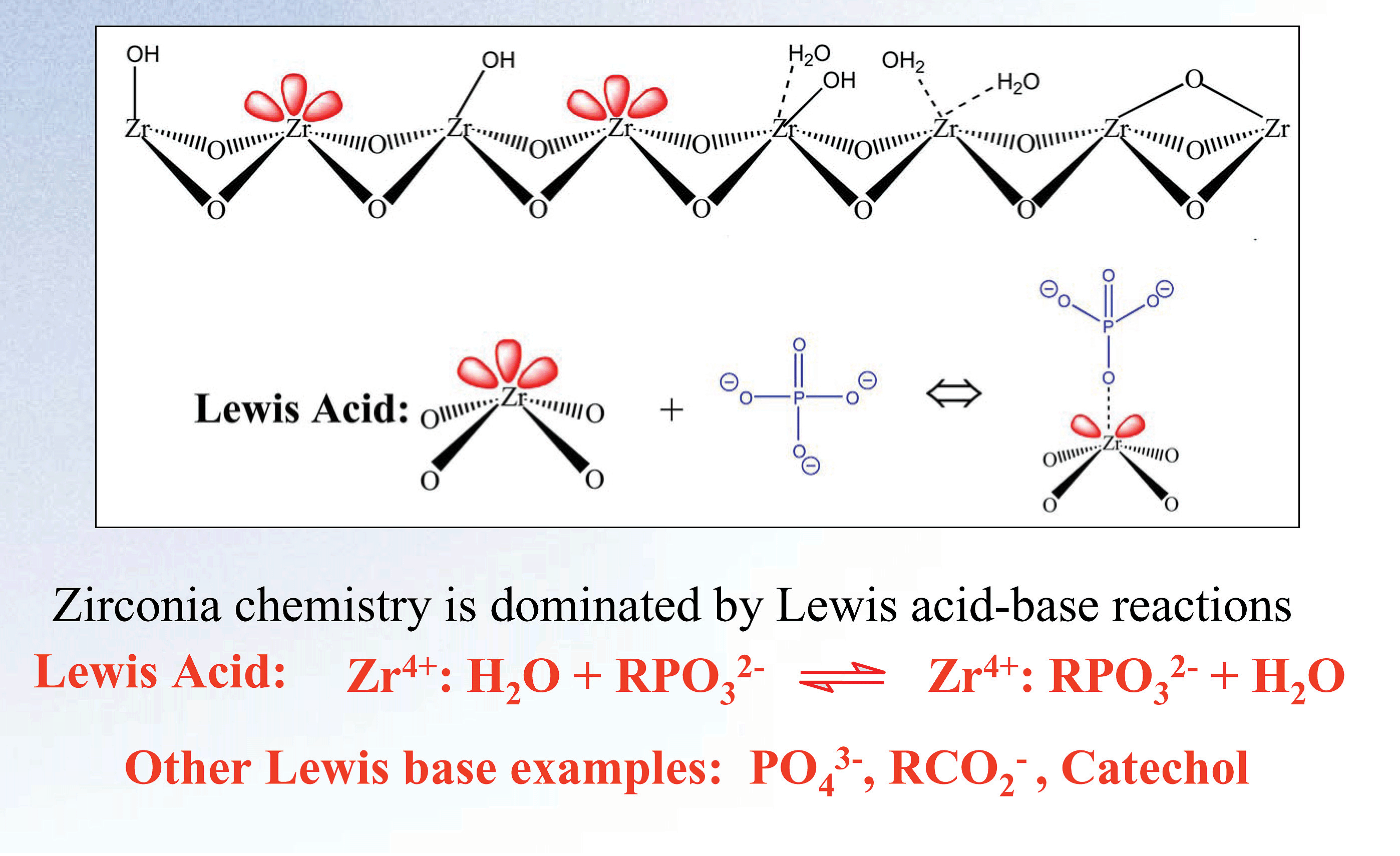
Zirconia's basic structure offers unparalleled chemical and thermal stability. The crystalline structure of zirconia has seven bond valency when compared to silica's four. The additional bonds in the crystalline structure of zirconia protect the structure from the hydrolysis that causes silica to degrade at low and high pH. The result of this unique configuration is a particle with superior pH (1-14) and temperature (up to 200║C) stability. This structure also enables a unique surface chemistry. The surface of zirconia has highly reproducible Lewis acid, Bronsted base, and Bronsted acid sites (see Figure 1).

Figure 1. Surface Chemistry of Zirconia
Although the benefits of zirconia's unparalleled stability are clearly apparent, the more subtle value of the multi-modal nature of zirconia may in fact be the most important factor when considering a zirconia column for your application.
ZIRCHROM«-PBD & ZIRCHROM«-MS COLUMNS - MULTI-MODAL SEPARATION OF CATIONIC ANALYTES (STRONG CATION EXCHANGE [SCX] & REVERSED PHASE [RP])
The ZirChrom«-PBD phase is coated with cross-linked polybutadiene (see Figure 2). The ZirChrom«-MS phase is similar to the ZirChrom«-PBD phase but the Lewis acid sites on the surface have been permanently deactivated with ethylenediamine-N,N,N',N'-tetra(methylenephosphonic) acid (EDTPA).
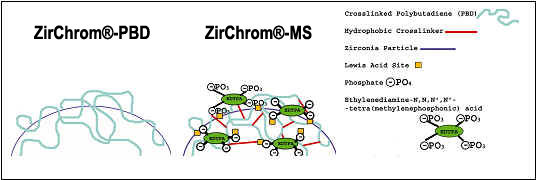
Figure 2. Surface Chemistry of ZirChrom«-PBD & ZirChrom«-MS
The Lewis acid sites on the surface of ZirChrom«-PBD allow for a tunable, multi-modal, selectivity. Without the addition of any Lewis base modifiers in the mobile phase the surface of zirconia is positive at low pH, neutral at neutral pH and negative at high pH (See Figure 3).
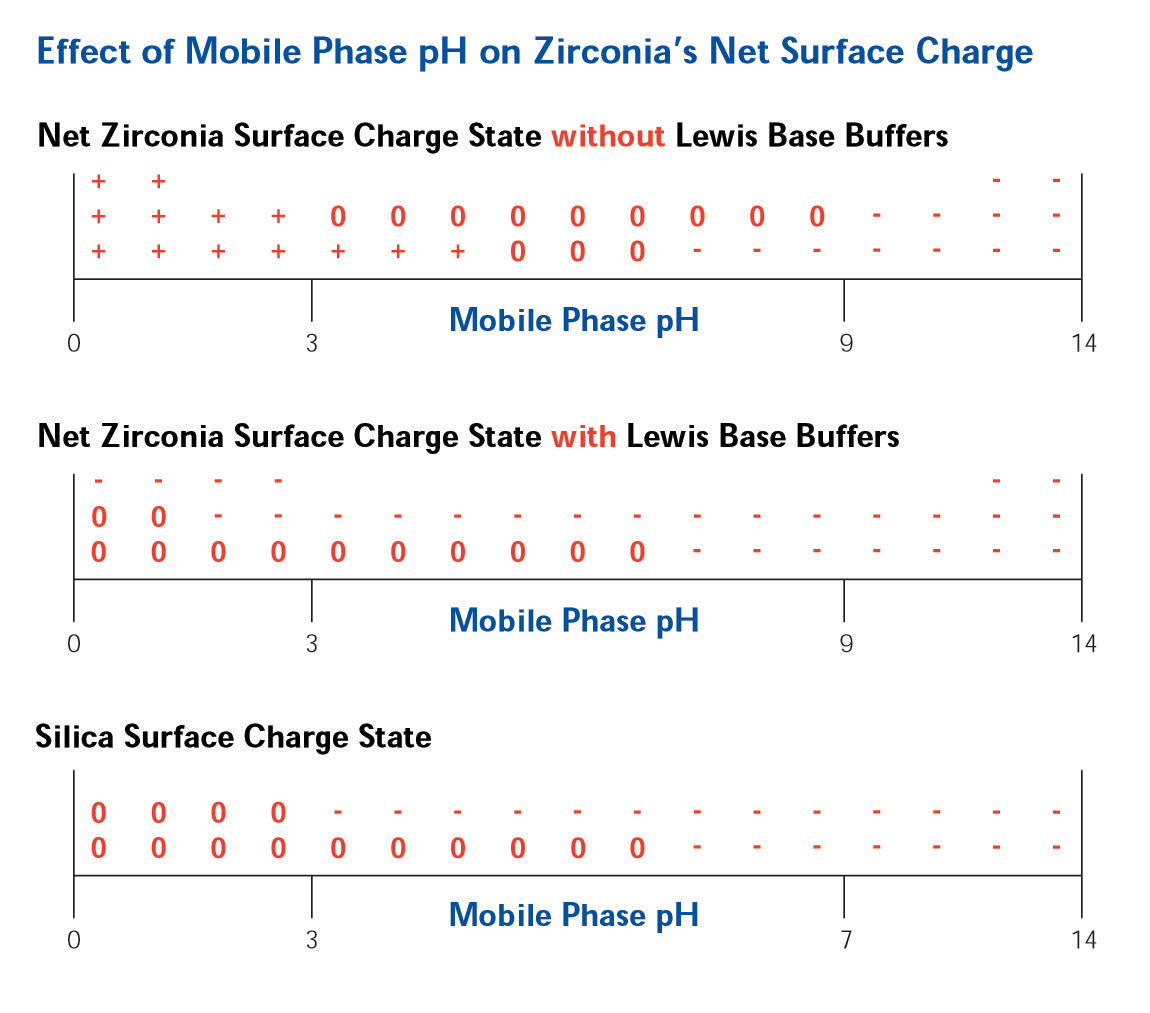
Figure 3. Effect of Mobile Phase pH on Surface Charge
The addition of a Lewis base modifier (such as carboxylate or phosphate) to the mobile phase will cause the modifier to adsorb to the zirconia surface in a ligand exchange interaction with the Lewis acid sites on the surface. The magnitude of the net negative charge imparted on the surface is directly correlated with the strength of the Lewis base modifier on the elutropic scale (See Figure 4).
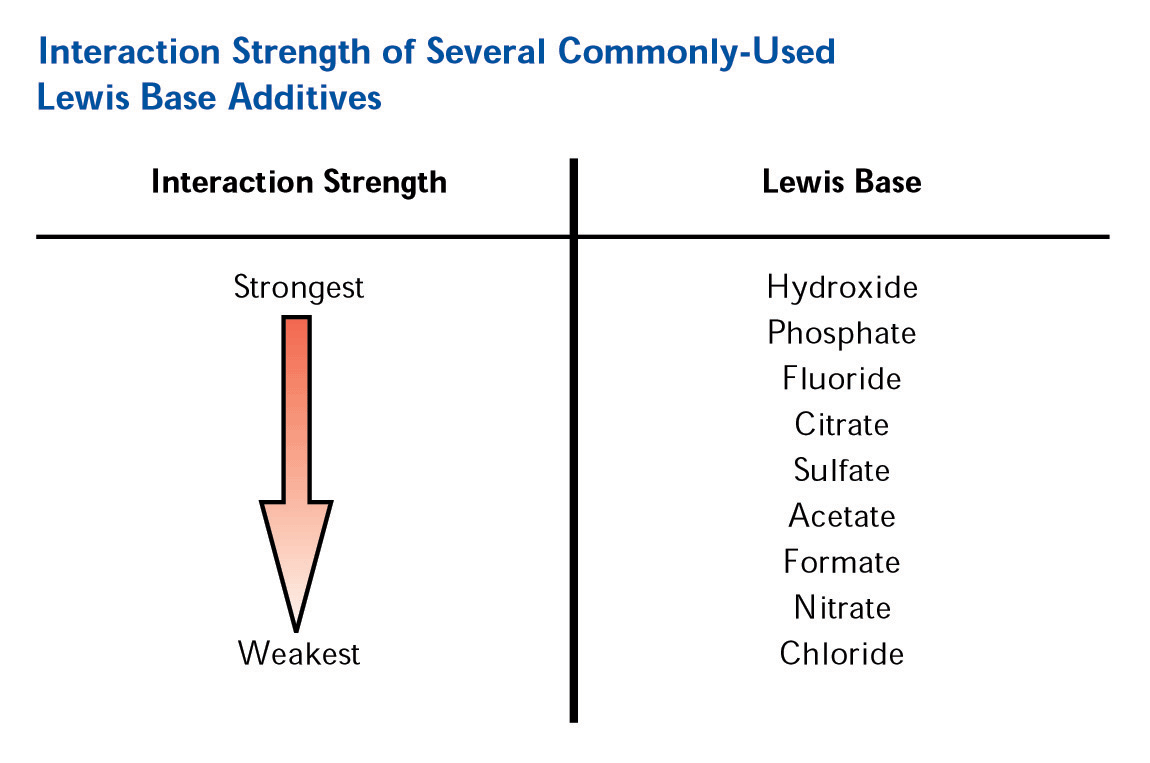
Figure 4. Elutropic Scale for Lewis Base Modifiers
The negative charge imparted on the surface (even at low pH with phosphate or EDTPA) coupled with the reversed phase character of the polymer coating and unparalleled thermal and chemical stability allows for a very unique multi-mode separation by cation exchange and reversed phase. Furthermore by judiciously selecting the mobile phase modifier and separation pH, the extent each mode (SCX or RP) plays in the separations can be controlled (see Figure 5). This enables users to "tune" the separation, allowing for effortless optimization of the analysis.
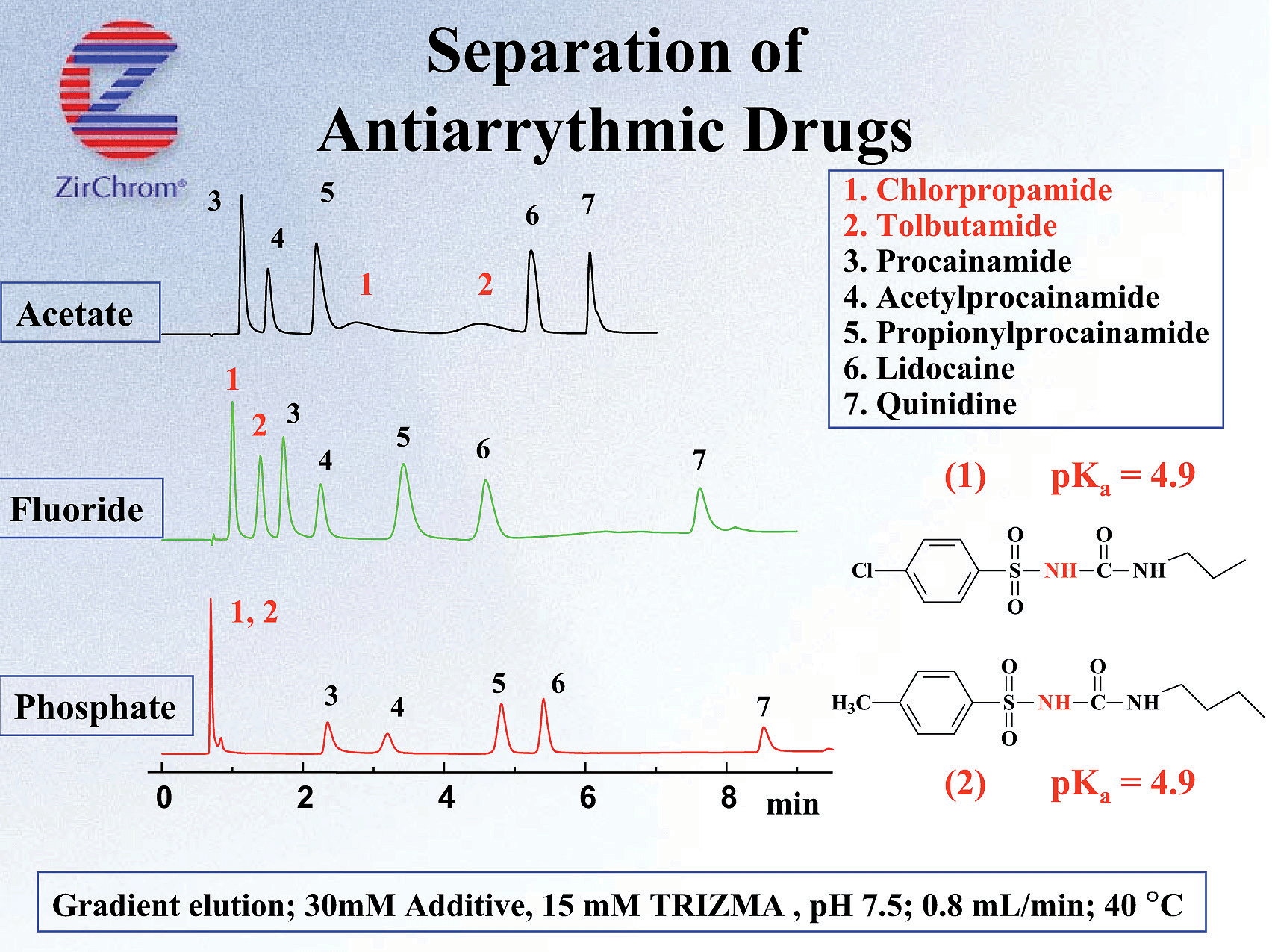
Figure 5. Effects of Lewis Base Modifier on the Retention of Antiarrythmic Drugs on ZirChrom«-PBD (TRIZMA - Tris(hydroxymethyl)aminomethane)
ZIRCHROM«-SAX - MULTI-MODAL SEPARATION OF ANIONIC ANALYTES (STRONG ANION EXCHANGE [SAX] & REVERSED PHASE [RP])
ZirChrom«-SAX is a cross-linked polyetheleneimine coated zirconia particle used for strong anion exchange, (see Figure 6). The hydrophobic cross-linker, shown in red, imbues the phase with a hydrophobic character and thus a multi-mode interaction. Although the dominant modes of selectivity for the ZirChrom«-SAX phase are anion exchange and reversed phase, the analyst still needs to be aware that the Lewis acid sites on the surface of the zirconia are available for interaction with Lewis base analytes. When analyzing compounds which are Lewis bases, a Lewis base modifier of equal or higher strength should be added to the mobile phase to achieve optimal peak shape and separation.
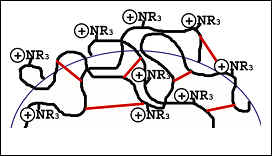
Figure 6. Surface Chemistry of ZirChrom«-SAX
The multi-modal nature of this phase lends itself especially well to the analysis of biologicals and nutraceutical products, (see Figure 7).
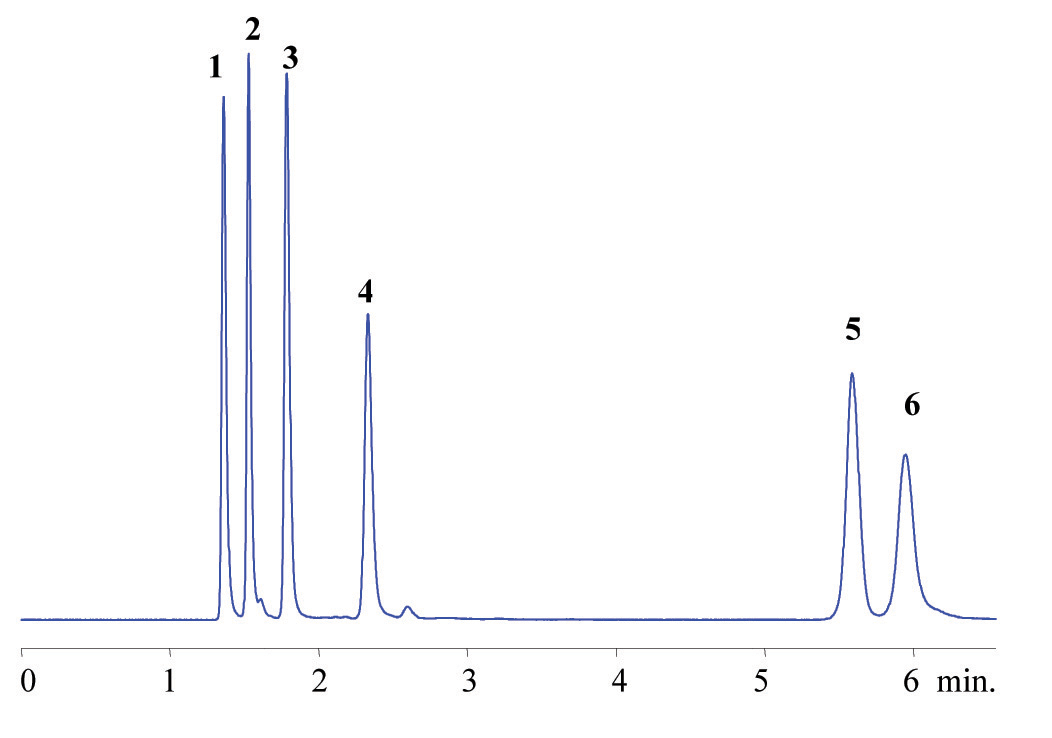
Figure 7. Analysis of Water-Soluble Vitamins on ZirChrom«-SAX. Separation of (1) Thiamine, (2) Pyridoxine, (3) Nicotinamide, (4) Riboflavin, (5) Nicotinic acid, and (6) Ascorbic acid on ZirChrom«-SAX, 150 x 4.6 mm i.d. (P/N ZR06-1546); Mobile Phase: 50 mM Ammonium dihydrogenphosphate, pH 4.5; Flow Rate: 1.0 ml/min; Temperature: 30 degrees C; Detection by UV @ 254nm. Technical Bulletin # 305.
ZIRCHROM«-CARB - UNIQUE SELECTIVITY FOR DIASTEREOMERS, ISOMERS & OTHER CLOSELY RELATED COMPOUNDS
ZirChrom«-CARB is crafted by coating zirconia with a very thin monolayer of elemental carbon. The resulting phase is flat, lacking a partitioning mode of separation, but allowing for enhanced shape selectivity for closely related compounds, especially where structural differences affect pi bonding between the compound and phase. This column is particularly ideal for diastereomers and geometric isomers, (see Figure 8). The selectivity of this phase often observed to be orthogonal to a typical silica C18 column making it an excellent choice for closely related or highly polar compounds. However, in order to achieve the optimum performance, special considerations must be made when selecting a mobile phase. These considerations are detailed in our Reversed Phase Method Development Guide.
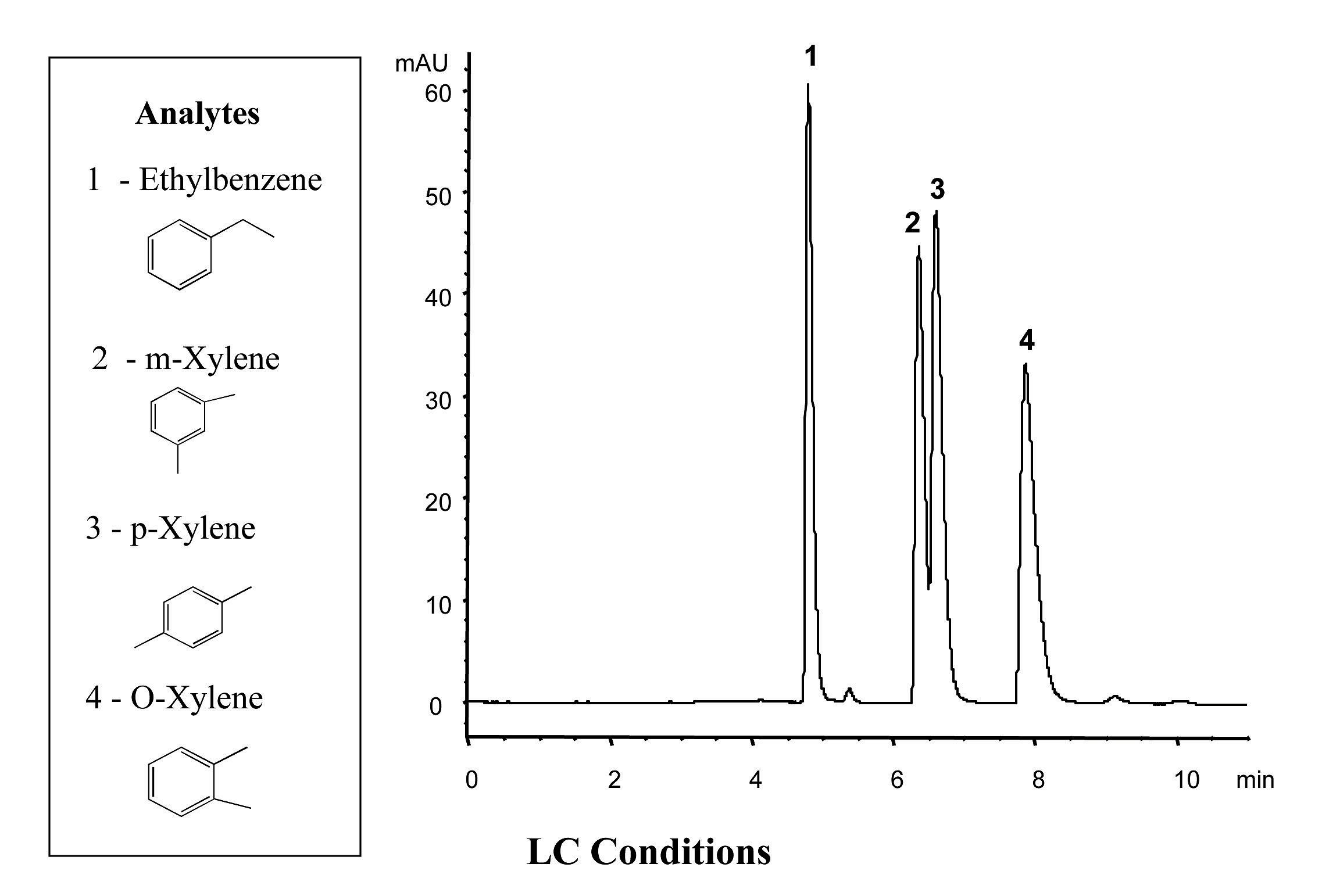
Figure 8. Isomer Separation on ZirChrom«-CARB. Separation of (1) Ethylbenzene, (2) m-Xylene, (3) p-Xylene, and (4) O-Xylene on ZirChrom«-CARB, 150 x 4.6 mm i.d. (P/N ZR01-1546); Mobile Phase: 50/50 Acetonitrile/Water; Flow Rate: 1.0 ml/min; Temperature: 30 degrees C; Detection by UV @ 254nm. Technical Bulletin # 134.
Full application notes for the separations in this newsletter as well as additional examples of multi-mode separations are available on our website at: http://www.zirchrom.com/ or by subscribing to our Z-APPS electronic application notes. Our next installation of Z-APPS will feature our recent multi-modal research.
Additional information regarding ZirChrom« HPLC products is available on the ZirChrom« website at: www.zirchrom.com or by subscribing to our Z-APPS electronic application notes. Our next installation of Z-APPS will feature our recent multi-modal research.
Please visit our booth, #222, at EAS 2008 and ask for the special trade show discount offer.