ZirChrom Newsletter vol. #10
Vol.# 10- “Sachtopore-NP and Sachtopore-RP, titania-based HPLC phases” (PDF)
Welcome to the tenth issue of ZirChrom's electronic newsletter. This newsletter introduces our titania-based HPLC phases, Sachtopore-NP and Sachtopore-RP. These new phases combine the increased thermal and chemical stability traditionally associated with zirconia-based phases with a wider range of pore and particle sizes.
WHAT IS SACHTOPORE?
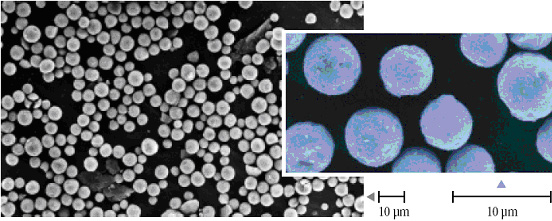
Sachtopore is an inorganic, porous, HPLC stationary phase comprised of titanium dioxide (titania). The particles are spherical, monodisperse and are available in a wide range of well controlled particle and pore sizes. Figure 1 below shows a scanning electron micrograph of porous Sachtopore titania particles.

Figure 1. Scanning electron micrograph of Sachtopore 100A, 5um (1) The surface chemistry of titania is quite different from the more traditional silica and polymer stationary phases, but is closely chemically related to zirconia. Like zirconia, the dominant surface features of titania includes: Brönsted acid, Brönsted base, and Lewis acid sites (see Figure 2).
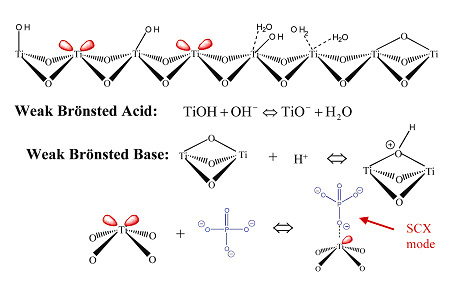
Figure 2. Surface Chemistry of Titania (Sachtopore-NP) (2)
Currently Sachtopore is available in normal and reversed-phase modes: Sachtopore-NP and Sachtopore-RP, respectively. Sachtopore-NP is a bare titania particle whereas Sachtopore-RP is polyethylene coated titania (see Figure 3). Both stationary phases are very chemically and thermally stable and offer unique chromatographic selectivity.
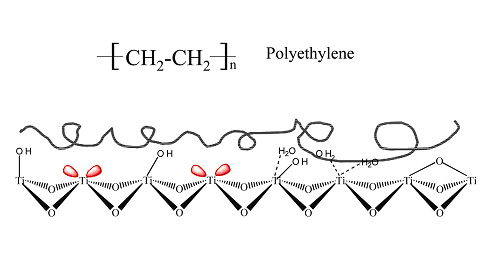
Figure 3. Surface Chemistry of Sachtopore-RP (2)
The Lewis acid site on the surface of titania plays a significant role in the chromatography of electrolytic analytes. The selectivity of the titania phases can be tuned by the ionic strength and type of buffer used (See Figures 4 & 5). These effects are predictable and follow the strength of Lewis acid-base interaction between the titania surface and the buffer. A stronger interaction with the titania results in a higher negative surface charge of the particle and greater retention of cationic solutes. The ionic retention mechanism can be reduced through the addition of salt to the mobile phase as shown in Figure 5 below.
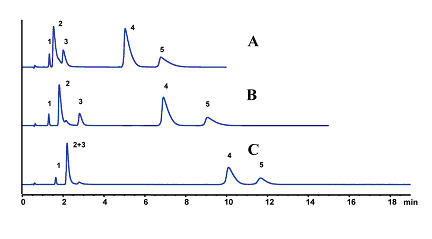
Figure 4. Effect of Lewis Base Additive on Separation of Basic Drugs. LC Conditions; Column: Sachtopore-RP (300A, 3um), 50 x 4.6 mm i.d. [part# TI01-0546-3(300)], Mobile phase: 30/70 ACN/20 mM buffer (pH=7), (A) ammonium acetate, (B) ammonium fluoride, (C) ammonium phosphate, Flow rate: 1.0 ml/min, Temperature: 40° C, Detection: UV, 254 nm, Solutes: (1) lidocaine, (2) quinidine, (3) tryptamine, (4) amitriptyline, and (5) nortriptyline. (2)
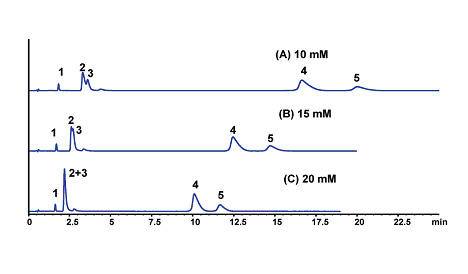
Figure 5. Effect of Ionic Strength on Separation of Basic Drugs. LC Conditions; Column: Sachtopore-RP (300A, 3um), 50 x 4.6 mm i.d. [part# TI01-0546-3(300)], Mobile phase: 30/70 ACN/phosphate buffer (pH=7), (A) 10 mM, (B) 15 mM, (C) 15 mM, Flow rate: 1.0 ml/min, Temperature: 40° C, Detection: UV, 254 nm, Solutes: (1) lidocaine, (2) quinidine, (3) tryptamine, (4) amitriptyline, and (5) nortriptyline. (2)
Table 1 compares select physical and chemical properties of titania and zirconia. Both titania and zirconia have a large particle size availability, however only titania has a modifiable pore size. Despite this major difference the balance of physical and chemical properties are equivalent. The relative surface area (taking into account differences in specific gravity and pore size), coordination number and isoelectric point are all similar in magnitude. Titania is both chemically and thermally stable.
|
|
TiO2 |
ZrO2 |
|
Porous particle size range(um) |
3 – 80 |
3 – 25 |
|
Average pore diameter (Å) |
Modifiable (60 Å, 100 Å, 300 Å, 500 Å, 1000 Å,
2000 Å) |
300Å |
|
Crystalline modification |
Anatase (60, 100, 300 A pore) - Rutile (2000 A pore) a |
monoclinic b |
|
Coordination number |
6 a |
7 b |
|
Isoelectric point (pH) |
5.6 a |
6.7 b |
|
pH stability range |
1-14 (NP) a 1-12 (RP) |
1-14 (PBD, CARB, PS, DB-C18)) |
|
Maximum temperature |
Up to 100C |
Up to 200C (CARB, DB-C18) |
a - Ref 1., b - Ref 3.
Table 1. Chemical and Physical properties of titania versus zirconia.
WHY CHOOSE SACHTOPORE?
The Sachtopore phases combine the increased stability and unique selectivity traditionally associated with zirconia-based phases with the versatility of a wide range of pore and particle sizes.
STABILITY
The Sachtopore-RP material is shows no loss of retention or efficiency using pH range of 1-12 and temperature up to 100°C (see Figure 6 & 7). The Sachtopore-NP material (bare titania) shows no degradation using a pH range of 1-14, even using 1M NaOH as an eluent (1).
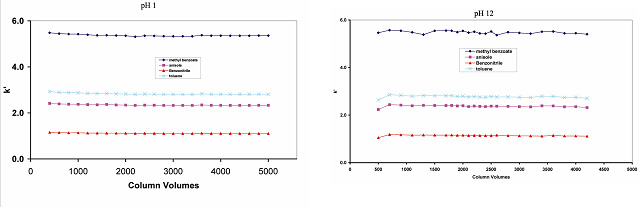
Figure 6. Chemical stability of Sachtopore-RP. LC Conditions; Column: Sachtopore-RP (300A, 3um), 50 x 4.6 mm i.d. [part# TI01-0546-3(300)], Mobile phase: 15/85 ACN/0.1M Nitric acid, pH 1.0, or 0.01M Tetramethylammoniumhydroxide, pH 12.0, Flow rate: 1.0 ml/min, Temperature: 30° C, Injection volume: 5 ul, Detection: UV, 254 nm. (2)
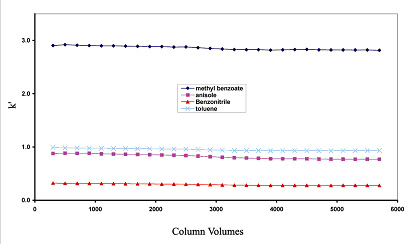
Figure 7. Temperature stability at 100°C of Sachtopore-RP. LC Conditions; Column: Sachtopore-RP (300A, 3um), 50 x 4.6 mm i.d. [part# TI01-0546-3(300)], Mobile phase: 15/85 ACN/water, Flow rate: 1.0 ml/min, Temperature: 100° C with Metalox heater, Injection volume: 5 ul, Detection: UV, 254 nm. (2)
SELECTIVITY
A selectivity comparison utilizing a set of 22 non-electrolyte solutes were analyzed using Sachtopore-RP, ZirChrom-PBD, and a standard silica-C18 column. No major selectivity differences between the ZirChrom-PBD and Sachtopore-RP columns (see Figure 8. retention data normalized to benzene).
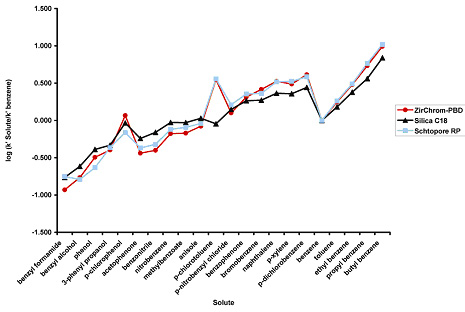
Figure 8. 22 Non-electrolyte Solute Selectivity comparison. LC Conditions; Column: Sachtopore-RP (300A, 3um), 50 x 4.6 mm i.d. [part# TI01-0546-3(300)], Mobile phase: 40/60 ACN/Water, Flow rate: 1.0 ml/min, Temperature: 30° C, Injection volume: 5 ul, Detection: UV, 254 nm. (2)
VERSITILITY
The Sachtopore particle is highly versatile; allowing modification of both particle and pore size. A particle size range of 3 - 80 um and a pore size range of 60 - 2000A is currently available (see Figure 9).

Figure 9. Scanning Electron Micrograph of 60, 100, 300, 2000 porous Sachtopore particles. (4)
The stability and versatility of the Sachtopore phases make it ideal for large, production scale separations that could greatly benefit from the ability to use highly alkaline or acidic mobile phase or cleaning-in-place procedures. An example of the scalability of the product is shown in Figure 10. To simulate preparative chromatography conditions, Pentifylline, a vasodiliator, was extracted from a tablet of CosaldonTM. Using 10 mM Na2B4O7 + 1 mM H3BO3 / 20% Acetonitrile (pH 9 in aqueous component) as mobile phase the chromatogram shown in Figure 10 was obtained under overload conditions. The Pentifylline peak appeared with an almost rectangular shape.
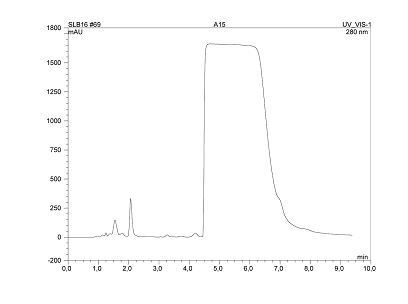
Figure 10. Preparative separation of Pentifylline. Impurities were separated at retention time of 1.55, 2.07 and 4.22 minutes. LC Conditions; Column: Sachtopore-RP (300A, 3um), 150 x 4.6 mm i.d. [part# TI01-1546-3(300)], Mobile phase: (+ 10 mM Na2B4O7 + 1 mM H3BO3), pH 8.8, Flow rate: 1.0 ml/min, Temperature: ambient, Injection volume: 20 ul, Detection: UV, 254 nm. (5)
RESEARCH & APPLICATIONS
Titania is on the cutting-edge of biological, pharmaceutical, and normal phase applications method development.
BIOLOGICAL
Historically, researchers have been unable to fully realize the benefits of mass spectrometry as an analysis method for phosphopeptides because isolation of the molecules from non-phosphorylated peptides is frequently required before examination of the complex samples can proceed (6). Previously, immobilized metal affinity chromatography (IMAC) was the most widely utilized technique for phosphopeptide enrichment by mass spectroscopy. IMAC methods can vary widely in effectiveness depending on the type of metal ion and loading/elution procedure. The technique also uses valuable research time for the required metal ion loading and washing steps and is difficult to incorporate into an on-line application (6). As non-specific binding of non-phosphorylated peptides further hampers the technique, researchers using mass spectroscopy needed a more specific on-line technique for isolating the phosphopeptides in order to fully realize the time saving benefits of mass spectroscopy (6, 7). Recently, several papers have been published demonstrating the unique ability of titanium dioxide (titania) to selectively retain phosphopeptides contained in complex biological mixtures (6, 7).
For example, Pinkse et al. report and innovative approach to automate the method for the enrichment of phosphopeptides using a 2D technique with titania dioxide particles as the first dimension and a reversed phase silica C18 column as the second dimension (6). The complex proteolytic digests were loaded onto the titanium dioxide pre-column using acidic conditions; retaining the phosphorylated peptides and allowing the rest of the digest to concentrate on the reversed phase column. After the analysis of the digest peptides on the reversed phase column is complete the phosphopeptides are eluted, under alkaline conditions, from the titanium dioxide for analysis. The authors report the method has a recovery above 90% and allows for the identification of previously uncharacterized phosphorylation sites (6). Additionally, they report the titania pre-columns could be used for over 200 runs without reduced performance (6). However, using this method the titania does appear to have non-specific binding issues especially of nonphosphorylated peptides with acidic residues.
Larsen et al. took the Pinkse research one step further and dramatically improved the selectivity of the Pinkse method by loading the peptide samples onto the titania in 2,5-dihydrobenzoic acid (DHB) (7). In a direct comparison of the titania and IMAC methods for semi-complex samples the titanium dioxide pre-columns had a greater yield of phosphorylated peptides and fewer contaminating non-phosphorylated peptides (7). This effect was enhanced as the complexity of the samples increased (7).
PHARMACEUTICAL
Following are two of the many excellent examples of using reversed-phase titania for the separation of pharmaceutical compounds. Sachtopore-RP delivers excellent peak shape and selectivity for basic pharmaceuticals.
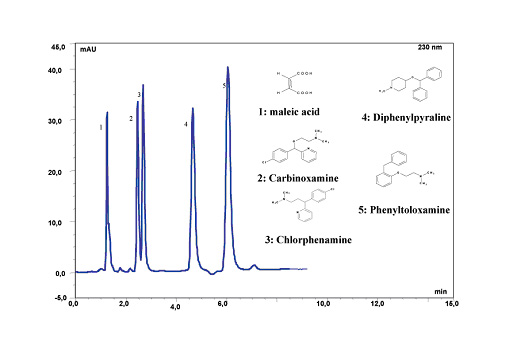
Figure 11. Separation of Anithistamines at pH 10. LC Conditions; Column: Sachtopore-RP (300A, 3um), 150 x 4.6 mm i.d. [part# TI01-1546-3(300)], Mobile phase: 74% (50 mM H3PO4 + 5 mM KH2PO4), 26% ACN, pH 10, Flow rate: 1.0 ml/min, Temperature: ambient, Injection volume: 20 ul, Detection: UV, 220 nm. (5)
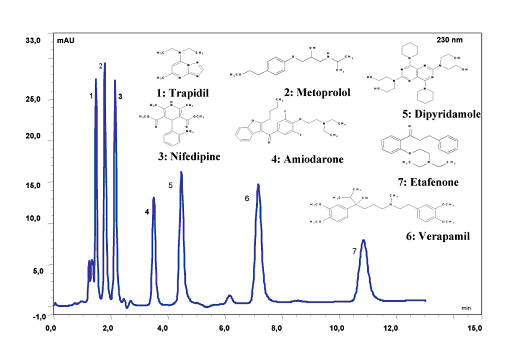
Figure 12. Separation of Basic Cardiac Drugs at pH 10. LC Conditions; Column: Sachtopore-RP (300A, 3um), 150 x 4.6 mm i.d. [part# TI01-1546-3(300)], Mobile phase: 70% (10 mM Borax + 10 mM Soda), 30% ACN, pH 10, Flow rate: 1.0 ml/min, Temperature: ambient, Injection volume: 20 ul, Detection: UV, 230 nm. (5)
NORMAL PHASE
The unique crystalline structure and surface chemistry of Sachtopore-NP enables the separation in normal phase of many basic and isomeric compounds (Figure 13 & 14).
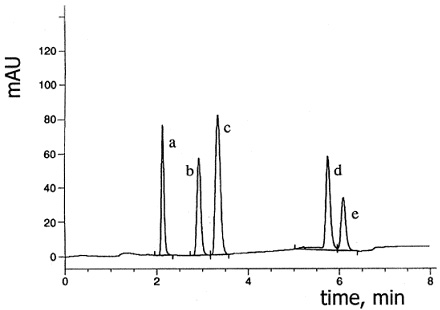
Figure 13. Separation of isomers of chloraniline in gradient elution on Sachtopore-NP. LC Conditions; Column: Sachtopore-NP (100A, 5um), 150 x 4.6 mm i.d. [part# TI02-1546-5(100)], Mobile phase: n-heptane / 2-propanol, gradient elution from (99/1) to (19/81) in 4 min, Flow rate: 1.5 ml/min, Temperature: 43° C, Detection: UV, 245 nm, Solutes: (a) 2,3-Dichloroaniline, (b) 2-chloroaniline, (c) 2,4-dichloroaniline, (d) 3-chloroaniline, (e) 3,4-dichloroaniline. (1)
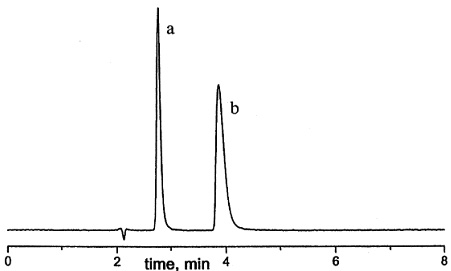
Figure 14. Separation of amitryptyline and noramitryptyline on Sachtopore-NP. LC Conditions; Column: Sachtopore-NP (100A, 5um), 150 x 4.6 mm i.d. [part# TI02-1546-5(100)], Mobile phase: dichloromethane / methanol [(TFA) (93:7) (0.2%), v/v], Flow rate: 1.0 ml/min, Temperature: ambient, Detection: UV, 254 nm, Solutes: (a) amitryptyline, (b) noramitryptyline. (1)
SUMMARY
Sachtopore is an inorganic, porous, HPLC stationary phase comprised of titanium dioxide (titania). The surface chemistry of titania is quite different from the more traditional silica and polymer stationary phases, but is closely chemically related to zirconia. The increased stability, unique selectivity and versatility of Sachtopore, both in reversed and normal phase modes, make it an excellent choice for cutting edge HPLC analytical and preparatory method development.
Currently Sachtopore offers two phases: Sachtopore-NP and Sachtopore-RP. Sachtopore-NP and Sachtopore-RP are available from ZirChrom in bulk or packed columns. Also available from Glygen are the Lab-in-a-Tip™ SPE pipette tips containing Sachtopore particles (Figure 15).

Figure 15. Glygen's Lab-in-a-tip™ SPE pipette tips
More information on Sachtopore bulk, packed columns, or Glygen's Lab-in-a-tip™ SPE pipette tips is available on our website at www.zirchrom.com or by contacting a ZirChrom technical specialist by phone at 1-866-STABLE-1 or by e-mail at support@zirchrom.com.
REFERENCES